Overview
The Division of Cardiology’s Clinical Trials Unit (CTU) is committed to supporting biomedical research endeavors to improve heart and vascular health outcomes. Practicing physicians, government agencies and industry sponsors alike rely on the CTU for central coordination of clinical studies at the University of Washington. We orchestrate the necessary people, services, and infrastructure to carry out responsible and effective research.
Activities
The CTU facilitates clinical investigations from start-up through close-out. Our work involves:
- Advising on study design and feasibility
- Contract and budget negotiation
- Institutional Review Board (IRB) submissions
- Administration of tests and procedures
- Federal, state, and institutional regulatory compliance
- Reserving clinic rooms and laboratory facilities
- Connecting sponsors with UW physicians
- Data and specimen collection
- Participant recruitment
- Shipping biological samples
- Consent development and screening
- Safety and results reporting
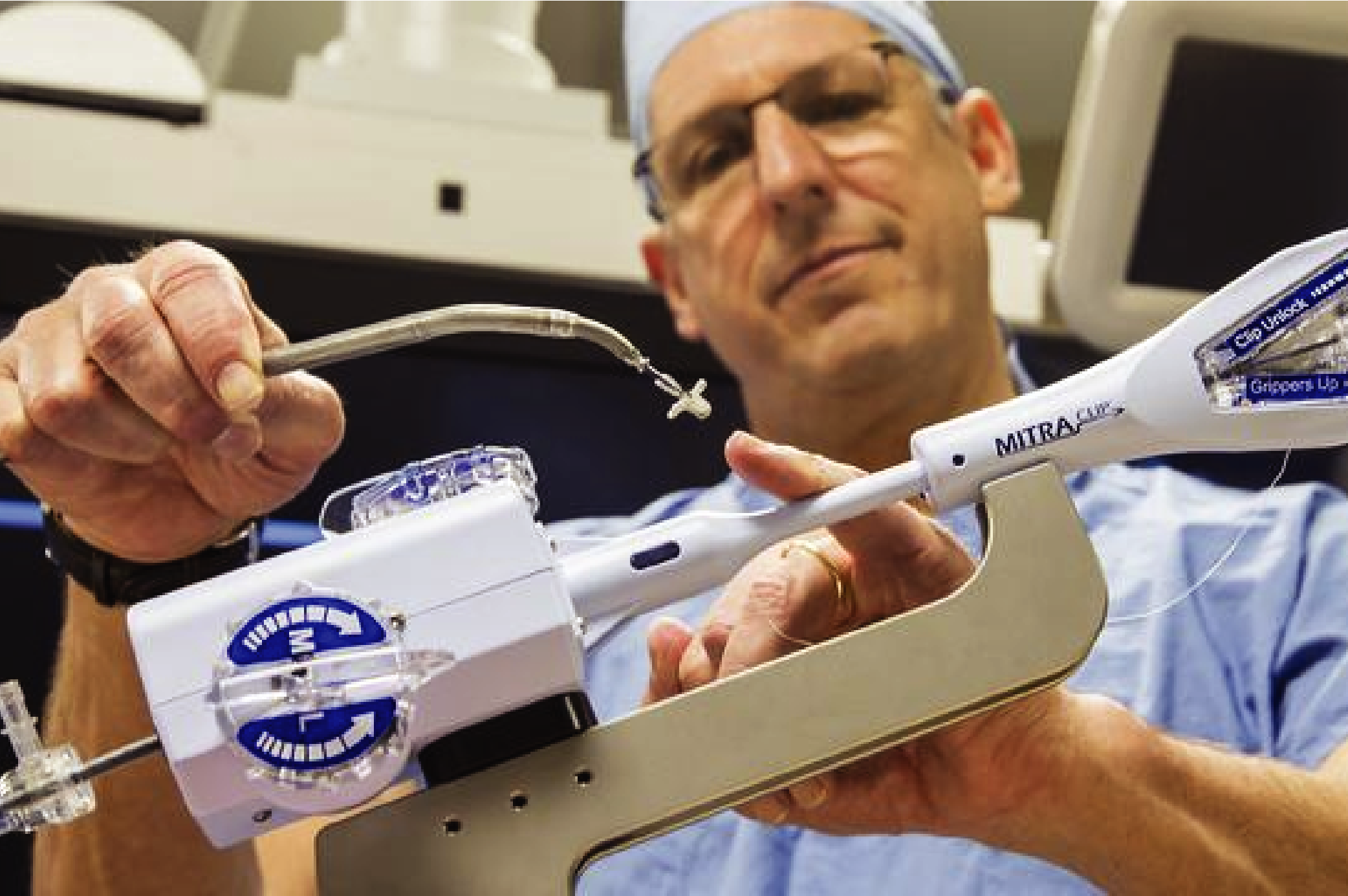
With our services, researchers can readily explore strategies for the detection, treatment and prevention of cardiovascular disease. The aim is often to determine the safety and efficacy of a new or modified therapy. Current projects are testing medications and devices to address problems such as heart failure and valvular dysfunction.
RESEARCH LEADERSHIP

Director
Cardiovascular Clinical Trials
CONTACT US
Connect with the team via email or call to speak with our Research Manager.
Email: CTU@cardiology.washington.edu
Phone: (206) 221-9154
If you have questions, are interested in participating, or want to sponsor a study at our site, let us know!