Overview
Congestive heart failure (CHF) is a major source of morbidity and mortality around the world. In patients with CHF, fibrosis–the accumulation of excess collagen–impairs both contraction and relaxation of the heart muscle and promotes lethal arrhythmias. Because cardiac fibrosis has classically been seen as an inexorable result of injury, there has been little progress in the field and thus, specific therapies for cardiac fibrosis nonexistent. However, our analysis of heart tissue in both mouse models and patients with CHF undergoing LVAD implantation and cardiac transplantation has shown that collagen production is dynamic and that immune cells such as macrophages play an important role in the regulation of cardiac fibrosis.
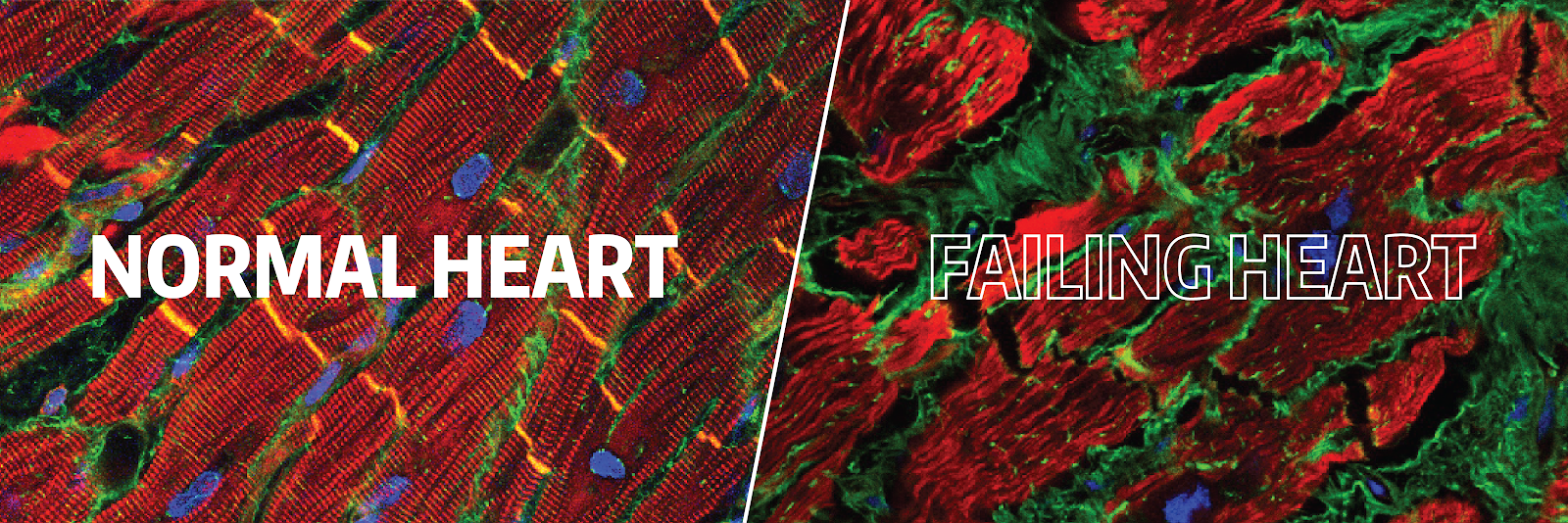
Goals and Approaches
The ultimate goal of our research is to design therapies to reverse cardiac fibrosis using:
- Translational studies in LVAD patients
- Mouse models of Fibrosis and Inflammation
- Cell therapy approaches to modulate inflammation
Current Projects
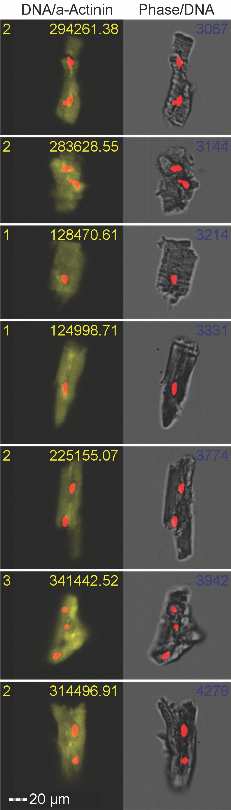 Modulation of Fibrotic Pathways in Patients with CHF
Modulation of Fibrotic Pathways in Patients with CHF
In these studies we are taking advantage of a “natural experiment” in which patients with advanced CHF require implantation of a mechanical heart pump, LVAD, to help them survive until a donor heart becomes available for cardiac transplantation. We collect heart tissue removed at the time of LVAD implantation and again at the time of cardiac transplant. Specific cells isolated from this tissue are then analyzed for changes in gene expression. We are exploring the effects of specific treatments on immune cells in the heart including nicotinamide riboside, a supplement that may decrease inflammation.
Engineered Macrophages to Treat Fibrosis
We are testing a cell therapy approach to fibrosis by using bioengineered macrophages. These immune cells can be delivered to the heart and using a chemical, “tuned” to an anti-fibrotic state. Our initial studies are in two mouse models of cardiac fibrosis, one from genetic manipulation and one from creating pressure overload via banding the aorta. Data from these mouse studies will allow us to refine this strategy with a goal to test the ability of engineered cells develop an engineered cells to decrease fibrosis in the LVAD patient model described above.
RESEARCH LEADERSHIP

Principal Investigator
April Stempien-Otero, MD
Associate Professor of Medicine
Cardiac Repair and Regeneration Lab
Email: april@uw.edu
Office: 206-616-9054